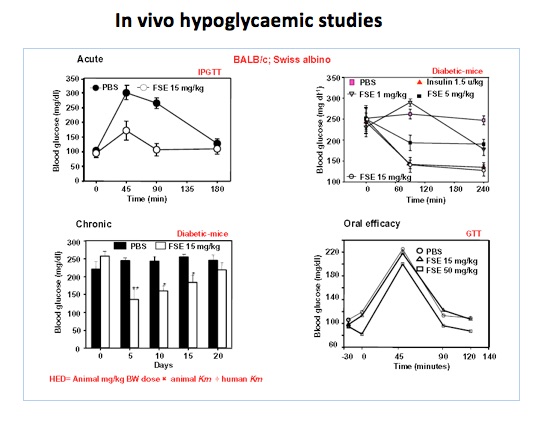
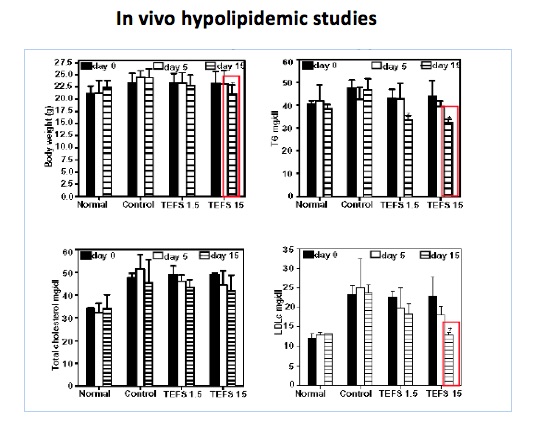
Fenugreek seeds are well known in Indian traditional medicine for use in the management of Diabetes and Obesity. However, the therapeutic dose of 25-50 g/day of Fenugreek seeds is not feasible for human consumption due to its bitter taste and pungent odour. Scientists at NCCS have designed and patented novel methods for preparing aqueous extracts of fenugreek seeds that would be reasonably convenient for human consumption. These extracts exhibit glucose and lipid lowering and weight lowering effects in animals at human equivalent dose of 75-150 mg/day. Preclinical studies have been performed to establish the safety of the extracts.
Technology Readiness: TRL B3 (In-vitro + In-vivo efficacy + Safety & Toxicology demonstrated)
Technology Status: PCT Filed (PCT/IN/2008/000877), Patents filed in India and Granted in USA (US8865237); and EU (2,323,676).
Technology Availability: Know-how available for transfer/ co-development with partners
Uploads: Scientist-pitch-format Presentation Dr MK Bhat 13-10-2015
References:
- The hypoglycaemic activity of fenugreek seed extract is mediated through the stimulation of an insulin signaling pathway. British Journal of Pharmacology, 2005, 146:41-45 (Article).
- Hypoglycemic effect of dialyzed fenugreek seeds extract is sustainable and is mediated, in part, by the activation of hepatic enzymes. Phytotherapy Research, 2008, 22:500-525 (Article).
- Hypolipidemic effect of fenugreek seeds is mediated through inhibition of fat accumulation and upregulation of LDL receptor. Obesity, 2010, 18:667-674 (Article).